
For example, d orbitals are made up of five degenerated orbitals that all have the same energy. Degeneracy will no longer exist in the system. In the presence of an external field, the degeneracy of the p orbital is unaffected however, the degeneracy of the f and d orbitals can be broken by applying an external field to the system (either electric or magnetic field).įew orbitals will have higher energy, while others will have lower energy. These orbitals are distinct (they may be oriented differently in space around the atomic nucleus), yet they have the same energy. Degenerate Orbitalsĭegenerate orbitals are those with the same energy. As a result, there are seven f orbitals for l = 3. The equivalent ml values for the f orbital are (-3,–2, –1, 0, +1, +2, +3). Because the value of l=3 for the f orbital, the minimal value of the primary quantum number n is 4.
Because the lobes are orientated along the x, y, or z-axis, they are given the names 2 px, 2 py, and 2 pz.

At the plane where the two lobes intersect, the likelihood of finding an electron is nil.
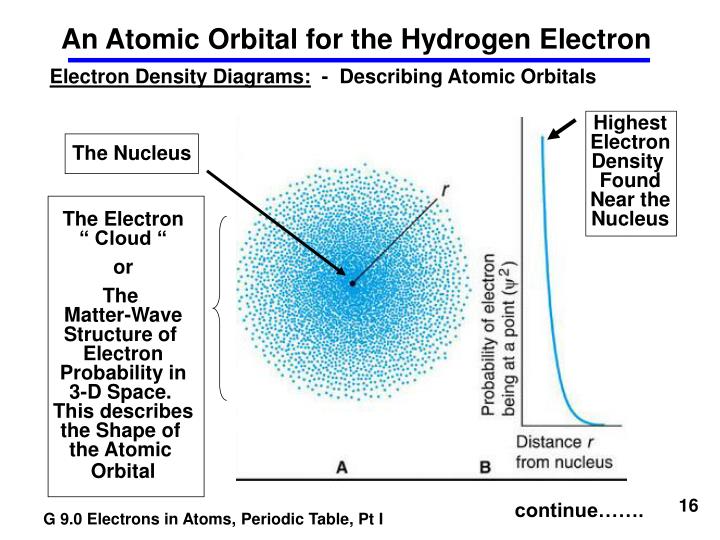
The distance from the nucleus is calculated by the radial nodes, while the orientation is determined by the angular nodes. Nodes are classified into two types: radial nodes and angular nodes. The nodal point is a location where there is no chance of locating the electron.The size of the s orbital is likewise shown to increase as the value of the primary quantum number (n) increases hence, 4s > 3s > 2s > 1s.S-orbitals are spherically symmetric, which means that the probability of finding an electron at a given distance is the same in all directions.The s orbital boundary surface diagram resembles a sphere with the nucleus at its centre, which can be shown in two dimensions as a circle.This wave function also aids in the creation of boundary surface diagrams. The likelihood of locating an electron is represented by the square of the orbital wave function. The orbital wave function, often known as is a mathematical function that is used to express the coordinates of an electron. A narrower orbital means there’s a better probability of catching an electron close to the nucleus. These orbitals can be classified based on their size, shape, or orientation. Augmented Assignment Operators in PythonĪn atom, according to the quantum atomic model, can have an infinite number of orbitals.Class 11 NCERT Solutions - Chapter 3 Trigonometric Function - Exercise 3.1.Class 11 NCERT Solutions - Chapter 7 Permutations And Combinations - Exercise 7.1.Difference Between Mean, Median, and Mode with Examples.What is the Difference between Interactive and Script Mode in Python Programming?.Molecular Nature of Matter – Definition, States, Types, Examples.ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.


 0 kommentar(er)
0 kommentar(er)
